
Stem cells are originator cells which include:
1. Hematopoietic Stem Cell (HSC)
Haematopoietic Stem Cells (HSC) are the stem cells which can replenish all blood cell types and self-renew. They can develop into red blood cells, white blood cells, and platelets. Red blood cells (erythrocytes) bring oxygen to all parts of the body, white blood cells (leukocytes) lock on to the germs in order to destroy them, and platelets (thrombocytes) help your body form blood clots and stop or prevent bleeding (D Bryder et., al.2006).
Haematopoietic Stem Cells (HSC) are obtained from bone marrow stem cells, peripheral blood stem cells, and umbilical cord blood stem cells, etc.

Figure 1. Blood Cell Development – how Haematopoietic Stem Cell (HSC) gives rise to all blood lineages.
Special Characteristics of Umbilical Cord Blood Stem Cells
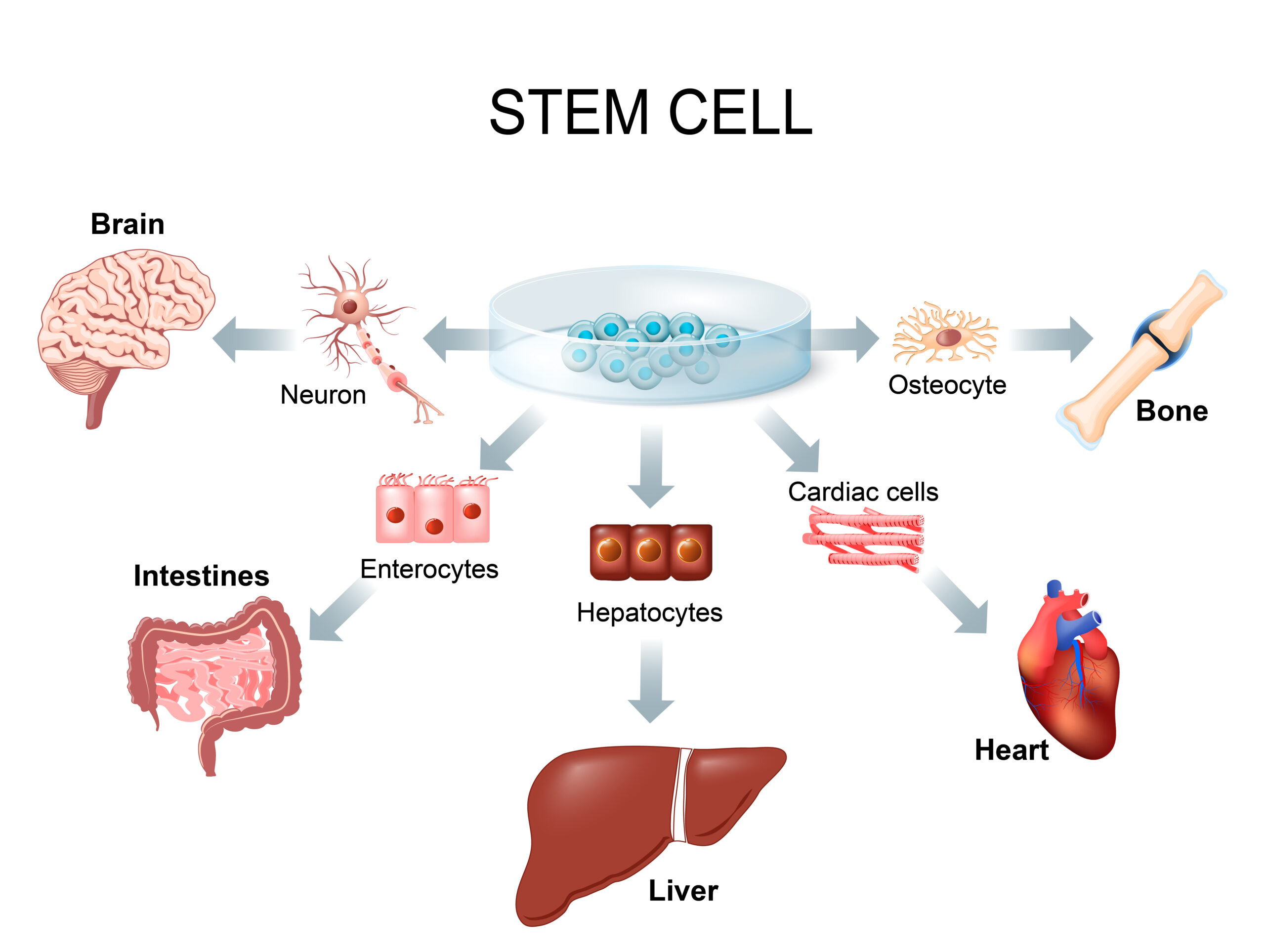
Umbilical cord blood stem cells can be used in the treatment of patients with the following diseases (S Roura et., al.2006).
- 1. Bone Marrow Disorders
- 2. Blood cancers i.e. leukemia or other types of cancers with chemotherapy
- 3. Congenital immunodeficiency disorders
- 4. Platelet disorders
- 5. Red blood cell disorders
- 6. Different types of Anemia with the need of regular blood transfusions
- 7. Thalassemia
- 8. Metabolic disorders
2. Mesenchymal Stem Cell (MSCs)
Mesenchymal stem cells (MSCs) have been studied and used clinically over the past 10 years due to an increase of degenerative diseases. These stem cells were first discovered and reported by A.J. Friedenstein et al.,1968. Since then a large number of clinical trials have been conducted, which consequently has made the researchers and the doctors studying these stem cells even more confident in applying them as clinical therapeutics. Moreover, the International Society for Cellular Therapy (ISCT) also established an official minimum criteria for MSCs in 2006 to standardize these stem cell treatments (Dominici M. et al.,2006) as follows;
1. MSCs must be the spindle shaped plastic-adherent cells.
2. MSC must express CD73, CD90, and CD105 and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules.
3. MSCs must differentiate to Osteoblasts, Chondroblasts, and Adipocytes in vitro.
Based on these criteria, MSCs must be cultured and checked for the special characteristics and ability to develop before use. The culture protocol and any procedure related to clinical application of MSCs needs to be addressed in clean environments, including Clean Room Class 100, and also in compliance with requirements of international standards e.g. ISO, GMP, GLP, etc. Most importantly, confirmation of functionality and safety of MSCs are needed before clinical application. Furthermore, an inspection of mold, bacteria, mycoplasma, and endotoxins contamination and sterility test must be conducted in accordance with GMP certified modern pharmaceutical manufacturing facilities.
Mesenchymal stem cells (MSCs) are multipotent adult stem cells isolated from many tissue sources with high safety and effectiveness. Studies show that MSCs constitutively express HLA-G at low level. As a result, the likelihood of cellular resistance between the donor and the recipient is extremely low, which is considered as new knowledge resulting from previous studies. However, MSC therapy is quite new in Thailand compared with Hematopoietic Stem Cells therapy which more experienced physicians can be found. Hence, all MSC therapies in Thailand must be conducted by specialized doctors or doctors certified by recognized organizations such as Cell Therapy Association of Thailand, etc.
References:
1. David Bryder, Derrick J. Rossi, and Irving L. Weissman. Hematopoietic Stem Cells. Am J Pathol. 2006;169(2): 338–346.
2. Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006;8:315–317.
3. Friedenstein A. J., Petrakova K. V., Kurolesova A. I., Frolova G. P. Heterotopic of bone marrow. Anal. Precursor Cells Osteogenic Hematopoietic Tissues. Transpl. 1968;6 230–247.
4. Jagannathan-Bogdan, M. & Zon, L. I. Hematopoiesis. Development, 2013;140:2463–2467.
5. Klyushnenkova E., Mosca J.D., Zernetkina V., Majumdar M.K., Beggs K.J., Simonetti D.W., Deans R.J., McIntosh K.R. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J. Biomed. Sci., 2005;12:47–57.
6. Santiago Roura, Josep-Maria Pujal, Carolina Gálvez-Montón, and Antoni Bayes-Genis. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther. 2015; 6(1): 123.
7. Mark L. Weiss and Deryl L. Troyer., 2006
Hi, this is a comment.
To get started with moderating, editing, and deleting comments, please visit the Comments screen in the dashboard.
Commenter avatars come from Gravatar.